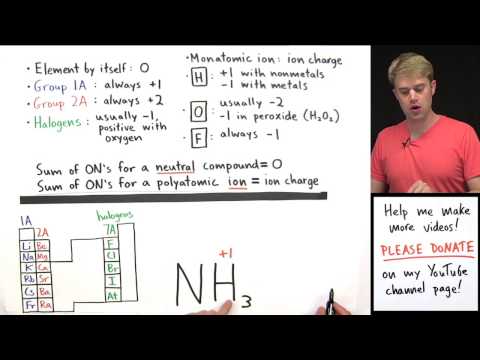
In this video, we're going to learn how to figure out the oxidation numbers for the different elements in a chemical compound. The oxidation numbers are the numbers that I've written here above each one of the elements. Now, if you want to learn more about what oxidation numbers are or why they're important, check out my video called "What are oxidation numbers?" In this video, we're going to work through the process of how you figure out what these numbers are. So, here are the rules that we're going to use to figure out oxidation numbers. Now, just so you know, every teacher and textbook has their own version of these rules, but they all work in pretty much the same way. So, if you learn mine, you'll still get the answer right 100% of the time, even if these are a little bit different from your teachers. I'm going to talk through a few of these rules right now, and then I'll introduce the rest just as we work through practice problems. The first rule is that an element by itself always has an oxidation number of zero. Here's what I mean by that. There are a lot of chemical compounds that have just one element, that element is not combined with any other elements. That's what I'm calling an element by itself. So, that's something like CL (-). It doesn't matter how many atoms of that element you have, just as long as it's only that element and none others. So, CL (-) has an oxidation number of zero. Another rule is about monatomic ions. These are ions that are made of only one element. So, for monatomic ions, their oxidation number is the same as their ion charge. For example, K+ has an oxidation number of...
Award-winning PDF software





Video instructions and help with filling out and completing How Form 2220 Compute