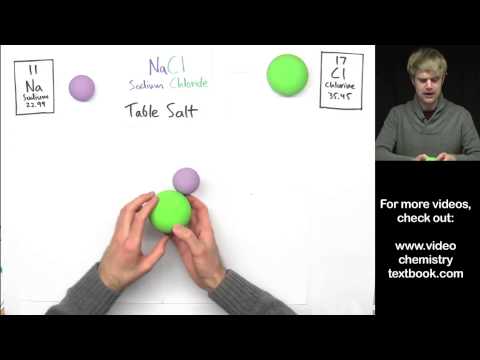
This video is an introduction to ionic bonds and ionic bonding. If you don't know anything about these things or you feel a little bit rusty, it's no big deal because we're going to start from scratch. So, ionic bonds are one type of chemical bond. Chemical bonds are like glue that holds atoms together. For example, here two atoms that are bonded together are connected, they're glued. Now, ionic bonds are the type of chemical bond that hold together metal atoms with nonmetal atoms. If you look at a periodic table, there's a big thick staircase that separates the metals, which are all the elements on one side, from the nonmetals, which are mostly elements on the other side. So, whenever we have a chemical that has a metal connected to a nonmetal, that's held together by ionic bonds. Some examples are silver chloride, magnesium iodide, or aluminum oxide. Each one of these chemicals has a metal from one side of the periodic table with a nonmetal from the other side, so ionic bonds are present in all of these compounds because there are metals and nonmetals connected together. Now, let's talk a little bit more about how and why these atoms connect together, what's holding them. To learn more about ionic bonds, we are going to focus on a chemical called sodium chloride, which is a fancy scientific name for table salt. Sodium chloride is made of two types of atoms: sodium and chlorine (or chloride). The atoms that I have right here are not glued together, they're just separate. So, I want to talk about what happens to get these separate atoms connected and glued together. The reason why these two atoms are connected is because they end up getting electrical charges. This atom is going to end up getting...
Award-winning PDF software





Video instructions and help with filling out and completing How Form 2220 Introductory