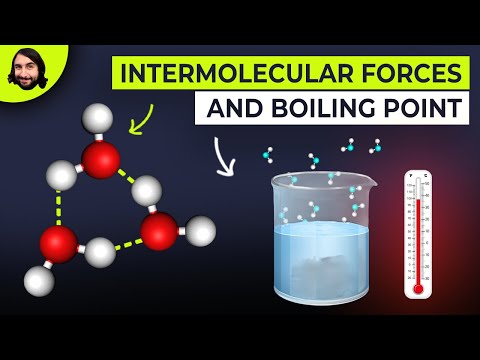
Professor Dave here. Let's talk about intermolecular forces. Dave explains what is happening when a liquid boils. Wider, different liquids boil at different temperatures. To answer these questions, we have to learn about intermolecular forces. These are the electrostatic interactions between molecules. So, atoms within a molecule make covalent and ionic bonds with each other, but molecules also participate in interactions with other molecules. Let's look at the different types. First, we have ion-ion interactions. Large ionic solids are held together by these networks of ionic bonds, which are the strongest intermolecular force because they involve formal charges. After that, we have ion-dipole interactions. So first, we must understand what a dipole is. The covalent bonds in a water molecule are polar because oxygen is more electronegative than hydronium and will pull the electrons in the bond towards itself. Because of the bent shape of the molecule, when we combine these vectors, we see water has an overall dipole, or a side of the molecule with some electron excess and a side with electron deficiency. Dipoles can make electrostatic interactions because the partially negative side is attracted to positive charges, and the partially positive side is attracted to negative charges. So, when sodium chloride dissolves in water, the sodium ions make ion-dipole interactions with the negative side of water's dipole, and the chloride ions make ion-dipole interactions with the positive side of water's dipole. Each ion can make several of these interactions, which store a lot of energy, which is why sodium chloride will dissociate in water in the first place. Next, we have dipole-dipole interactions. As you can guess, this is when dipoles interact with each other. As with pure water when in liquid form, water molecules will move in such a way so as to always be making...
Award-winning PDF software





Video instructions and help with filling out and completing What Form 2220 Respectively