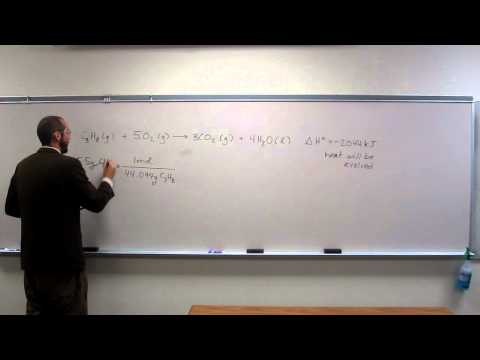Okay, anyway. So what is this problem? Say, how much heat, in kilojoules, is evolved or absorbed in the burning of fifteen point five grams of protein? Well, I guess we can figure out if it is going to be evolved or absorbed. How do you know that? This is a thing, right? So the equal sign means that they will balance each other out, like in the game "Leave the System to Play the Dinosaurs." Okay, so we're trying to figure out the number, or how much heat is involved in the burning of 15.5 grams of propane. So, we know the mass of propane, 15.5 grams, and we want to know the number of moles. So, let's change this to moles, and we know the molar mass, which is 44.94 grams/mol. Cancel, so that's one mole of C3H8. And what else do we know if we want to get energy up here, right? So, what conversion factor can we use? This is what you guys were saying, right? Like that? Because I got one mole over 44 grams. So now, all we have to do is plug in each other, and so it's going to be the three significant figures. So, 7.19 kilojoules of energy. Visible. Okay, cool. Let's go up to the lab, guys, and we'll continue this discussion.
Award-winning PDF software





Video instructions and help with filling out and completing Why Form 2220 Compute